Search
astrophysics (85)biophysics (18)chemistry (23)electric field (71)electric current (75)gravitational field (81)hydromechanics (146)nuclear physics (44)oscillations (57)quantum physics (31)magnetic field (43)mathematics (89)mechanics of a point mass (298)gas mechanics (87)mechanics of rigid bodies (221)molecular physics (71)geometrical optics (78)wave optics (65)other (167)relativistic physics (37)statistical physics (21)thermodynamics (154)wave mechanics (51)
thermodynamics
4. Series 23. Year - E. MacGyver and Thermometer
Construct a thermometer using the materials available at home. Base the calibration of the degree scale on well-known temperatures. Do not forget to attach a photograph of the end result of your efforts.
Honza Hermann (temporarily) went down with Parkinson
1. Series 23. Year - 3. adiabatic invariant
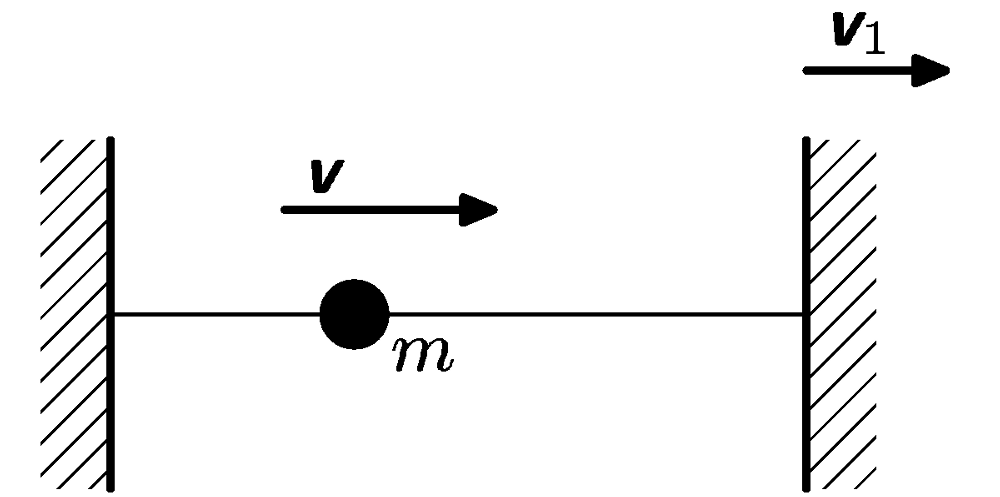
The movement of point mass is restricted on straight line by 2 end points. At the beginning the point mass $m$ is moving at speed $v$. The end point starts to move away at speed $v_{1}<<v$. How will the energy of point mass change?
Na Zajímavé teoretické fyzice nespala Janap.
1. Series 23. Year - E. antifreeze
We are going to the north pole. Having snowmobile helping to bring them north, we need into the cooler some antifreeze. What is the ideal concentration of alcohol in water, more precisely what is dependence of melting point on alcohol concentration? If you have good freezer, then measure, what concentration will freeze at some specific temperature.
Do not forgot, that this task is experimental.
Ze svých cest po Sibiři přivezl Jarda.
1. Series 23. Year - P. thermometer
The capillary of medical thermometer is at its bottom thinner, to stop mercury to return back to the reservoir at the base, which allows us to read the maximum reached temperature. From June 2009 it is forbidden to sell such thermometers. At this historical occasion, take the opportunity and explain us, why the capillary narrowing works only in one direction – only when heating. Because while cooling down the mercury cannot go through the narrow point back.
Při horečce chtěl podvádět Honza Prachař.
6. Series 22. Year - 4. stone on the piston
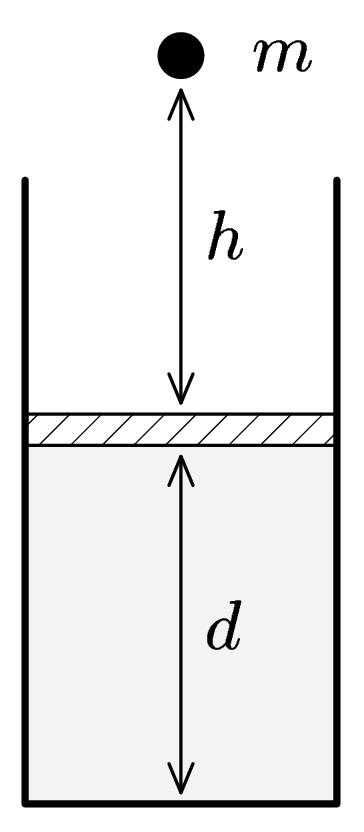
Marek has a piston of area $S$ filled with ideal gas in equilibrium ($p$, $V$ a $T)$. On this piston a stone falls down from the height $h$. Mass of stone is $m(see$ picture). Piston is pushed down and then comes up again and remains in a new position. How depends this new position on the mass of stone and the falling height? Is it possible for piston to become in equilibrium higher then the original position? How will change the temperature of gas?
vymyslel Mára po přednášce z termodynamiky
2. Series 22. Year - P. lovers under the duvet
How will the temperature increase, if there are two person under duvet instead of one.
vymyslel zmrzlý milovník Honza P.
6. Series 21. Year - 2. cooking in pressure cooker
Assume a pressure cooker, which is filled with half of water and remaining half is filled with air. Then it is closed that no water vapor or air can escape. The cooker with water is slowly heated. At what temperature will water start to boil? What phases are present inside the pressure cooker during increasing temperature?
Sbírka od Dalimila Mazáče.
4. Series 21. Year - 2. heating of a sphere
In this question we investigate influence of temperature on to moment of inertia of a metal body. Lets have an axe going through the body. How will change the moment of inertia $J$ when the temperature is increased by $ΔT$, if the coefficient of thermal expansion of metal is $α$. If you have problem with a arbitrary shape of body, consider sphere of cylinder.
V Havránkovi se úloha líbila Pavlu Motlochovi.
5. Series 20. Year - P. what type of windows?
One of organizers recently upgraded his windows in his house. They are now double-glazed. The space between two glass tables can be filled by inert gas or vacuum. Suggest a method to distinguish between these two types windows (without destroying windows).
Problém ze života Michaela Komma.
3. Series 20. Year - 4. Albert Einstein's heating
Albert Einstein in his retirement (in contrary to his peers working in the back gardens) still enjoyed solving difficult puzzles. In winter he noticed that water heated directly in fire gets war very slowly and the efficiency is small.
He decided to try other method: Take ideal heat machine and use boiler and air as hot and cold reservoir. Then use the gain work to another ideal heat engine which will get heat from air and transfer it to water. If the temperature of boiler, water and air is $T_{1}$, $T_{2}$ and $T_{3}$, what is efficiency of water heating? Does it conflict with second thermodynamic law?
Úlohu navrhl Matouš Ringel.