Search
astrophysics (85)biophysics (18)chemistry (23)electric field (71)electric current (75)gravitational field (81)hydromechanics (146)nuclear physics (44)oscillations (57)quantum physics (31)magnetic field (43)mathematics (89)mechanics of a point mass (298)gas mechanics (87)mechanics of rigid bodies (221)molecular physics (71)geometrical optics (78)wave optics (65)other (167)relativistic physics (37)statistical physics (21)thermodynamics (154)wave mechanics (51)
thermodynamics
(7 points)5. Series 31. Year - 4. thermal losses
At what temperature does the indoor environment of the flat in a block of flats stabilise? Consider that our flat is adjacent to other apartments (except its shorter walls), in which the temperature $22 \mathrm{\C}$ is maintained. The shorter walls adjoin the surroundings where the temperature is $ - 5 \mathrm{\C}$. The inside dimensions of the flat are height $ h = 2{,}5 \mathrm{m}$, width $ a = 6 \mathrm{m}$ and length $ b = 10 \mathrm{m} $. The coefficient of the specific thermal conductivity of the walls is $ \lambda = 0{,}75 \mathrm{W\cdot K^{-1}\cdot m^{-1}} $. The thickness of the outer walls and the ceilings are $ D\_{out} = 20 \mathrm{cm}$, and the thickness of the inner walls are $ D\_{in} = 10 \mathrm{cm}$.
How will the result be changed if we add polystyrene insulation to the building? The thickness of the polystyrene is $ d = 5 \mathrm{cm}$, and its specific heat conductivity is $ \lambda '= 0{,}04 \mathrm{W\cdot K^{-1}\cdot m^{-1}} $.
(3 points)4. Series 31. Year - 1. ice-cream
Estimate how many grams of ice-cream is possible to be made out of $5 \mathrm{l}$ of liquid oxygen with temperature $-196 \mathrm{\C }$ and unlimited amount of milk and cream with room temperature $22 \mathrm{\C }$? Let's suppose that ice-cream consists of milk and cream only (same mass of both ingredients) and the temperature of the ice-cream should be $-5 \mathrm{\C }$. Use average heat capacity $c\_m = 3{,}45 \mathrm{kJ\cdot kg^{-1}\cdot K^{-1}}$ for milk and $c\_s = 4{,}45 \mathrm{kJ\cdot kg^{-1}\cdot K^{-1}}$ for cream (despite the fact that changes considerably in this temperature range). Find other needed quantities on the internet by yourself.
Michal got a taste for ice-cream.
(3 points)1. Series 31. Year - 1. I'm gonna milk my coffee
When is it most efficient to pour cold milk into a hot coffee for the coffee to be drinkable as soon as possible? We expect a detailed description of the cooling process rather than a precise calculation.
Terka S. was baffled by the exclamation: „I've already put milk in your coffee to make it cool quicker.“
(7 points)1. Series 31. Year - 4. my bottle is cracking
What happens when we close an almost empty 1.5 liter PET bottle in a well-heated office, let's say at $t\_k = 26 \mathrm{\C }$, and then walk down the stairs with it? The bottle starts to make cracking noises. What has a bigger effect on this phenomenon, the fact the atmospheric pressure is changing as we walk down 10 floors of the building, or the fact that the temperature on the stairs is a bit lower, let's say, $t\_s = 15 \mathrm{\C }$?
Karel walked down the stairs in the faculty.
(7 points)6. Series 30. Year - 4. shoot your rat
Mirek wants to shoot a rat he sees at the dorm. To that end, he made a simple air gun which can be modeled as a tube with constant cross-section $S=15\;\mathrm{mm}$ and length $l=30\;\mathrm{cm}$ closed on one side and open on the other. Mirek plans to place a bullet of mass $m=2g$ into the tube so that the bullet seals to tube exactly and is fixed at a distance $d=3\;\mathrm{cm}$ from the closed end. He that pumps up the closed section to a pressure $p_{0}$ and then releases the bullet. He wants the speed of the bullet to be at least $v=90\;\mathrm{m}\cdot \mathrm{s}^{-1}$ as it exits the tube. What pressure will he need to achieve if the gas is ideal? Discuss the realism of the situation. Assume the bullet is released by a quasi-static adiabatic process where $κ=7⁄5$, as the gas is diatomic. Assume an external atmospheric pressure $p_{a}=10^5Pa$. Neglect losses due to friction, air resistance and gas compression ahead of the bullet.
Karel wanted to find out if the solvers could pass the Masters programme admissions at MFF
(9 points)6. Series 30. Year - P. evaporating asteroid
A very large piece of ice (let us say with diameter 1 km) is placed near a Sun-like star to a circular orbit. It is placed so close, that the equillibrium temperature of a black body at this distance would be approximately 30 ° C. What will happen with such an asteroid and its orbit? The asteroid is not tidally locked.
Karel likes astrophysics, so he came up with something again.
(8 points)4. Series 30. Year - 4. heat engine
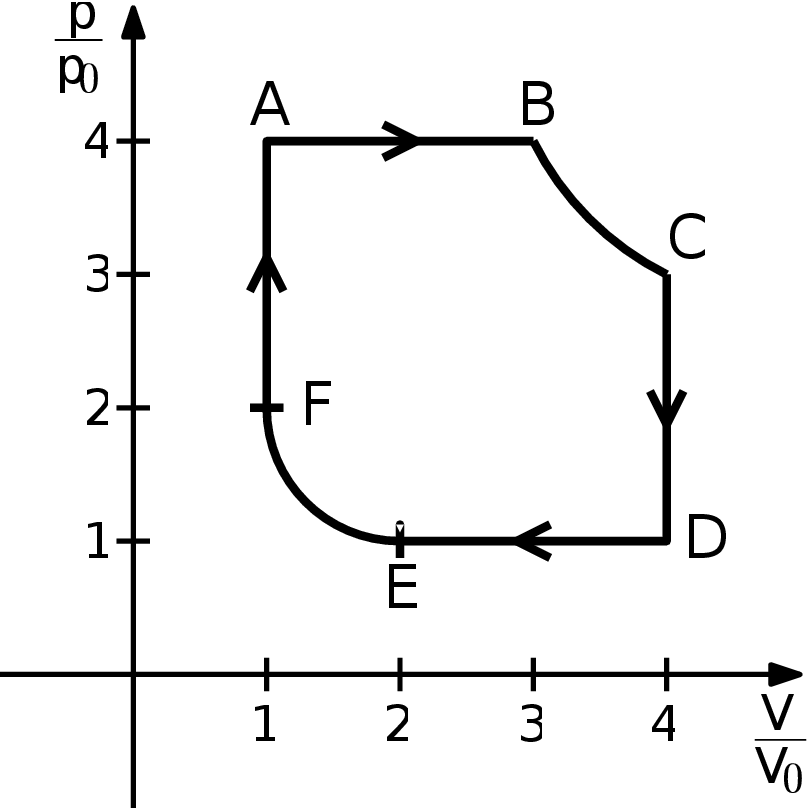
Consider a heat engine filled with a diatomic gas. This engine works thanks to a cycle ABCDEFA as shown in the picture. The 6 processes that make up the cycle are
- A $→$ B - isobaric heating from a state 4$p_{0}$ and $V_{0}$ (let us denote temperature at A 4$T_{0})$ to a state with volume 3$V_{0}$,
- B $→$ C - isothermic expansion to volume 4$V_{0}$,
- C $→$ D - isochoric cooling to pressure $p_{0}$,
- D $→$ E - isobaric cooling to volume 2$V_{0}$,
- E $→$ F - isothermic compression to volume $V_{0}$,
- F $→$ A - isochoric heating to pressure 4$p_{0}$. Determine the remaining state variables in B, C, D, E, and F, the maximal and the minimal temperature of the ideal gas during the process (as a multiple of $T_{0})$, heat received and lost by the gas in each process, and the overall efficiency of the engine. Compare this efficiency with that of a Carnot engine working between the same minimal and maximal temperatures. Assume for simplicity that the molar amount of the gas does not change and there are no chemical changes during the cycle. A sketch can be seen in figure.
Bonus: Do the same for a much simpler „square“ cycle, ABCDA, where the gas starts in a state $p_{0}$, $V_{0}$ and $T_{0}$ and izochorically heats up to 4$p_{0}$, isobarically heats up and expands to 4$V_{0}$, isochorically cools down to $p_{0}$ and isobarically cools down to $V_{0}$. Compare the efficiency of these two heat engines and suggest which one is better.
Karel was alternately warm and cold
(3 points)2. Series 30. Year - 2. ultra high temperature superconducticvity
Many types of materials, mostly metals, have increasing dependence of resistivity on temperature. However, there are semiconductors or graphite which show a decreasing dependence. And you have also probably heard about superconductivity, the natural phenomenon when a cooled material shows almost no electrical resistance and becomes a perfect conductor. Our current state of knowledge says that the temperature of a superconductor must be well below room temperature, but let's assume that the equation defining the resistance is $R=$ R_{0} (1 + αΔt)$$, where $R_{0}is$ the resistance at room temperature, $αis$ the temperature coefficient of resistance and $Δt$ is the temperature difference with respect to room temperature, and the equation holds for any temperature. Using this equation and coefficients $α_{C}=-0.5\cdot 10^{-3}K^{-1}$ for graphite and $α_{Si}=-75\cdot 10^{-3}K^{-1}$ for silicon, we obtain zero resistance for high temperatures. Determine these two temperatures and explain why the superconducting phenomenon does not work this way, i.e. neither carbon nor silicon are superconductors at high temperatures.
Karel se inspiroval nekonstantními konstantami.
(9 points)2. Series 30. Year - P. an effective machine
Guns can be considered to be heat engines. Calculate the efficiency of a gun, say a pistol or a rifle.
Michal původy svých nápadů raději nesděluje.
(3 points)1. Series 30. Year - 1. With rum, or without?
Three substances: water, steel and rum are put in a pot, which effectively doesn't convect any heat. The water has mass $m_{v}=0,5\;\mathrm{kg}$, temperature $t_{v}=90°F$ and specific heat capacity $c_{v}=1kcal\cdot \;\mathrm{kg}^{-1}\cdot K^{-1}$. The steel is in form of a cylinder, that has mass $m_{o}=200g$, temperature $t_{o}=60\;\mathrm{°C}$ and specific heat capacity $c_{o}=0,260kJ\cdot \;\mathrm{kg}^{-1}\cdot °F^{-1}$. Rum has mass $m_{r}=100000mg$, temperature $t_{r}=270K$ and specific heat capacity $c_{r}=3,5J\cdot g^{-1}\cdot \;\mathrm{°C}^{-1}$. What will be the temperature (in degrees centigrade) of the system when it reaches balance?
Lukáš Mirkovi sděloval svoje zkušenosti s alkoholem.